Regulatory compliance is getting more an more complex. We support you to start early and prepare thoroughly for document submission. Profit from our (CTIS) experience and well-established communication with the reviewing bodies.
Regulatory Affairs
Streamline the application process to obtain approval for your study in a short time frame
EU/EEA: Managing a clinical trial via CTIS
Submission via CTIS is mandatory since 31-Jan-2023
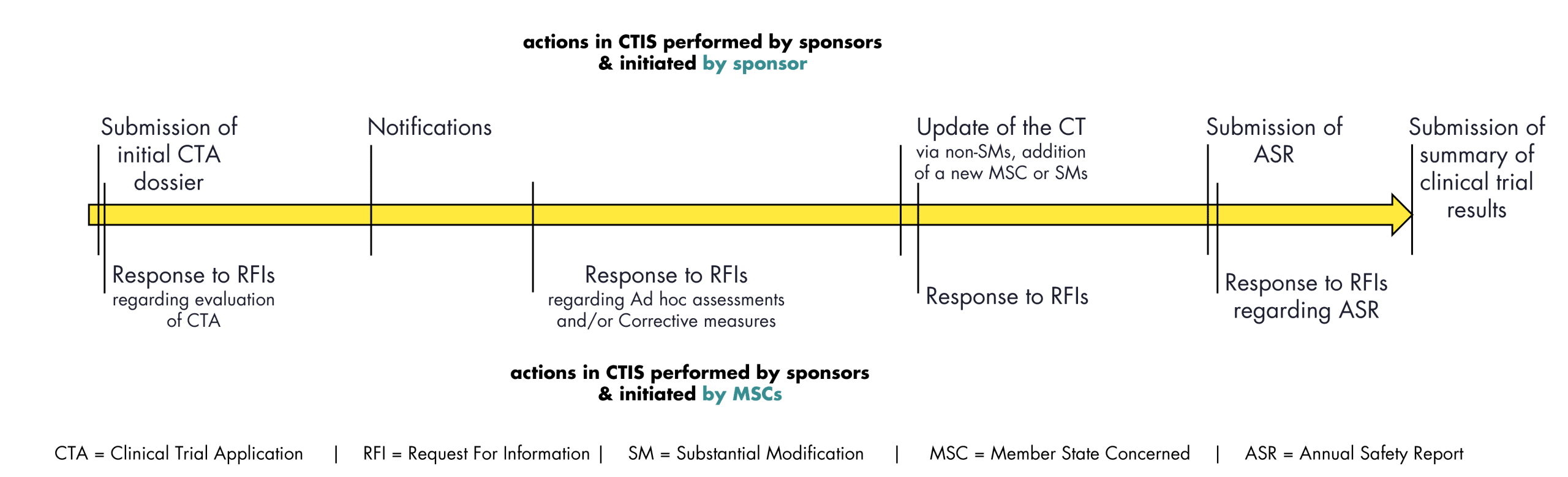
Streamlining the regulatory process
Our experience
First submissions of a CTAs via CTIS already performed and RFIs answered within the deadline.
Further submission of CTAs currently ongoing (Transfer & Initial)
CTIS submissions practiced thoroughly in test environment
ClinOps received basic training, RA SMEs are trained deeply
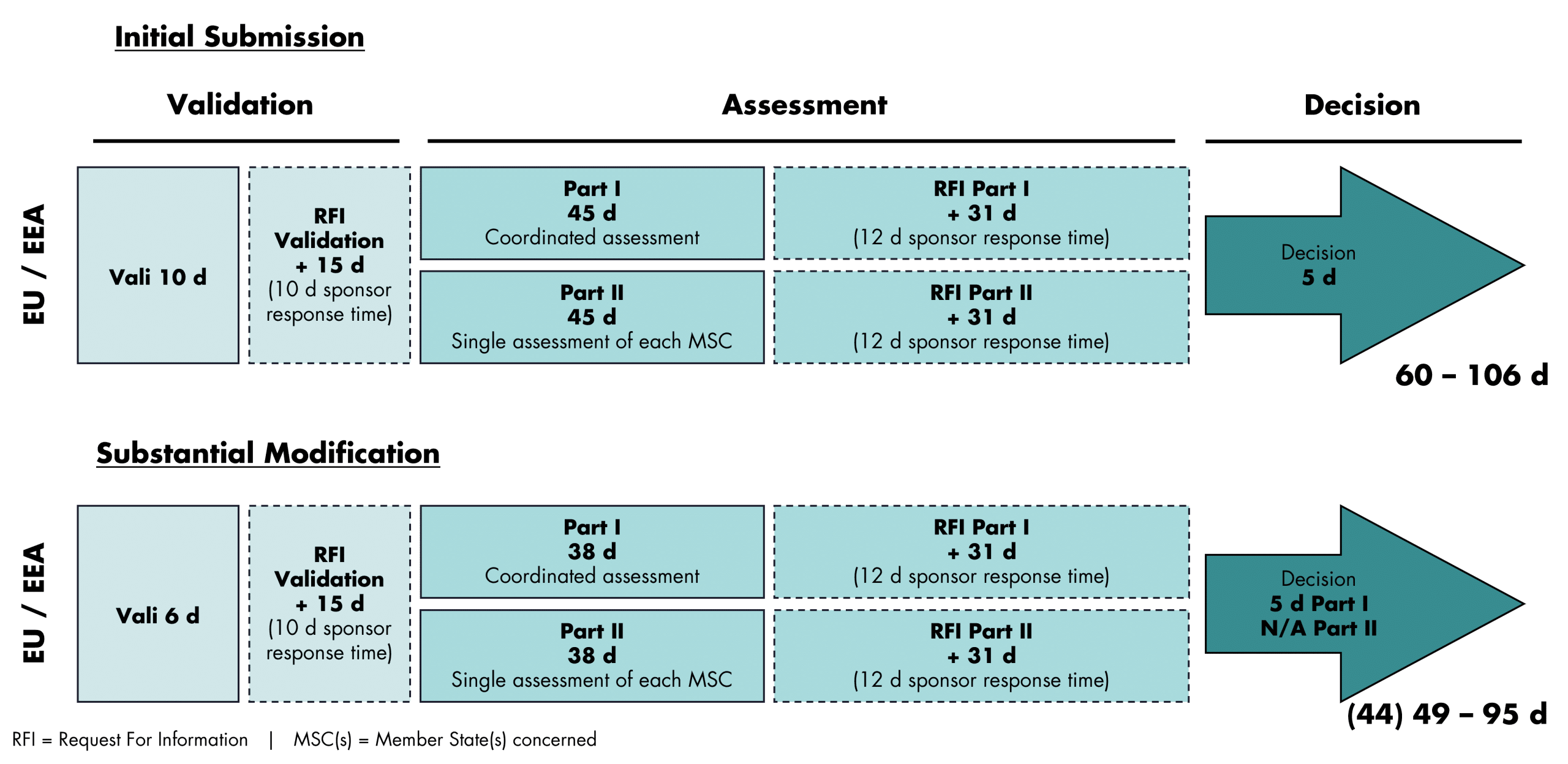
To shorten the assessment and approval timelines and to avoid unnecessary requests, the submission of a complete and high-quality dossier is of particular importance.
Avoid last-minute changes/ decisions.
Implement prospective requests, before MSCs ask for it.
Better add 1 week for planning, than waste 1 month for revision.
Have your SMEs available at critical timepoints.
Pick up the phone and ask us.
Regulatory Timelines CTIS
Contact us now and receive more information about our capabilities
Our Services
We are a full-service CRO. We take this literally – whether you choose all or some of our service functions.



