For a successfull clinical trial it is important to have a good data management system and collect accurate data as needed for your trial. Select the right system components tailored to project acquirements. Professional setup of your EDC system and train all neccessary stakeholder intensively. Moreover, it is important to continously clean the data to keep your records accurate and communicate with the study team.
Data Management & Biostatistic
Deliver accurate data for reliable clinical trial results
EDC Solution – System in-use and Feature
We do support various systems and all of our systems do feature
Browser based EDC solution (no software installation required)
Compliant with FDA 21 CRF part 11
Combined eCRF, ePRO, IRT solution
Independently build and validate databases
Automated import of external data (e.g. Lab data)
Automated and customizable export of data
Interactive reports and task lists

Process – eCRF Implementation
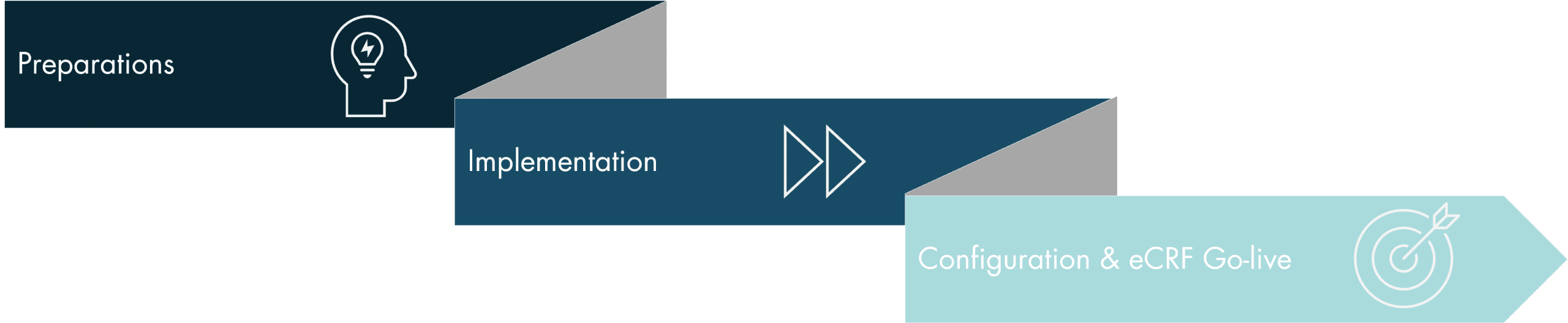
Setup of CRF development core team and eCRF roll-out plan
Early start of CRF design (CDISC CDASH)
Development of data transfer agreements with external data vendors
Full Data management documentation (DMP,DVP, UAT log…)
eCRF Implementation with user-friendly navigation
Randomization
Setup of interfaces for external data import
Online check and notification programming
Setup of data export
Setup of auto-encoding tool
Configuration of user roles and query workflows
Programming of custom reports
Setup of eLearning for eCRF users
ePRO Workflow
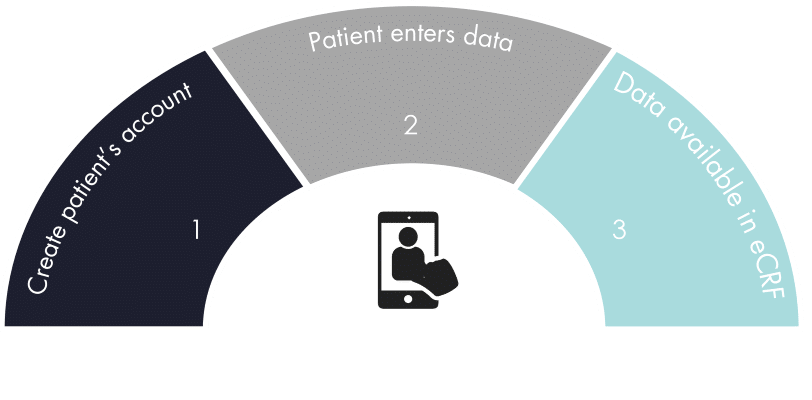
Integration of patient contact information
Patient identifying information is blinded to all other users
Patients receive notifications when data is expected
Form-level security controls access
Edit checks set allowable window for data entry
Data is immediately available in eCRF once ePRO is saved
Contact us now and receive more information about our capabilities
Our Services
We are a full-service CRO. We take this literally – whether you choose all or some of our service functions.



