Regulatory Update
Clinical trials under Regulation (EU) 536/2014 (Clinical Trial Regulation – CTR)
– A practical guide for sponsors, CROs, study sites –
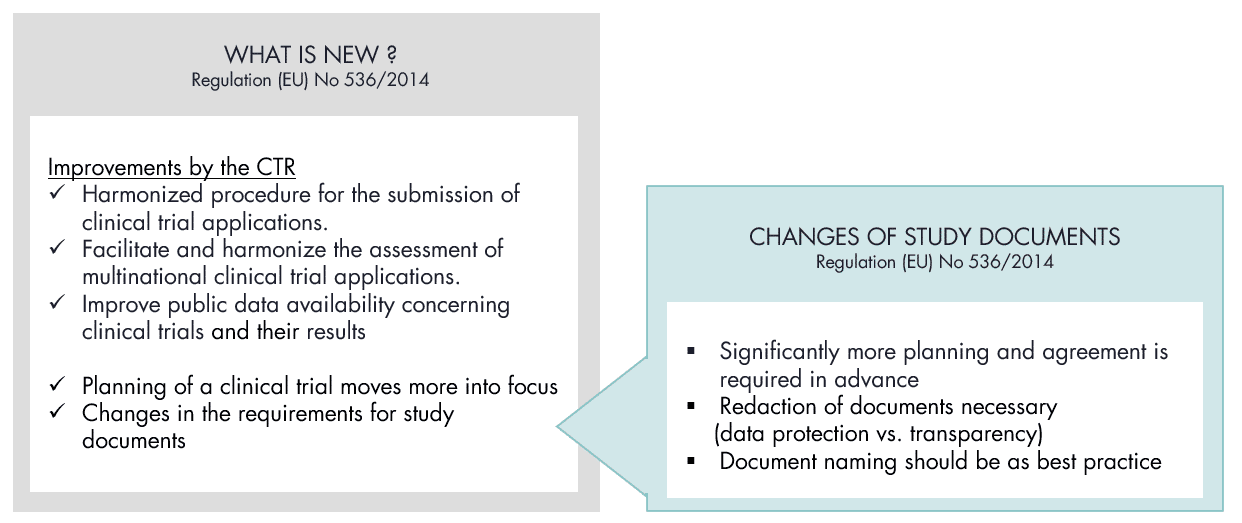
How the regulatory framework has changed
Our Service
Submission of initial CTA dossier
Patient Recruitment
How to find the right patients for your study.



